release date:2021-08-11
Recently, the whole chain of novel coronavirus nucleic acid detection products developed by TargetingOne has been granted CE market access in the European Union, providing two technical solutions for global anti-epidemic with excellent fluorescent quantitative PCR (qPCR) and digital PCR, which have outstanding advantages such as high sensitivity, accurate results and easy operation.
Novel Coronavirus(2019-nCov) detection products received the EU CE market access qualification

Full Chain Solution for Novel Coronavirus Testing
Home test, easy to use
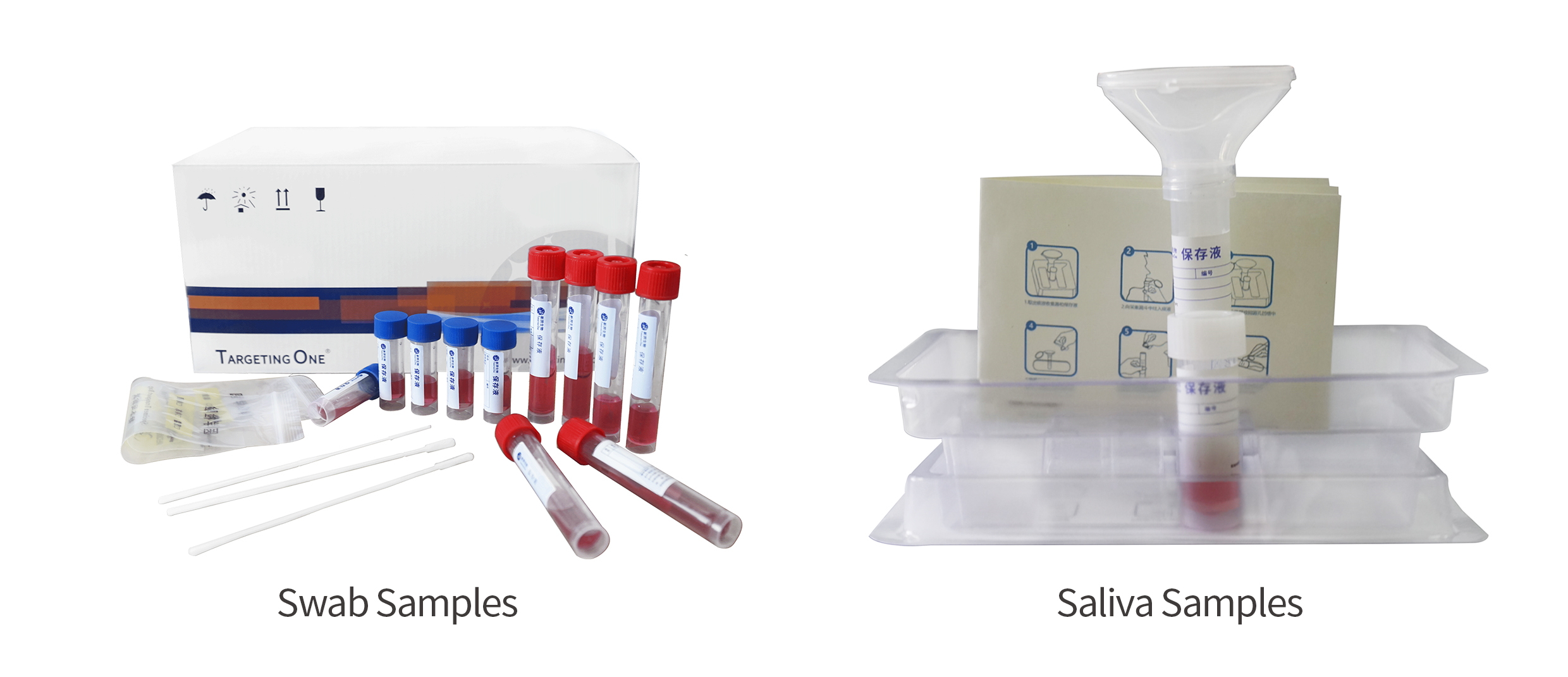
Higher biosafety, sampling and virus inactivation synchronization
Wide range of specifications and scenes to meet different sampling needs of customers
Effectively maintain virus activity, facilitate downstream immunization research
Effectively improve the detection rate of positive viruses and avoid false negatives
High extraction efficiency, low residue of magnetic beads
Precise temperature control, good temperature uniformity
High throughput, fast processing of multiple samples
Pre-encapsulated reagents, easy and fast to operate

Reagents are in lyophilized form: more stable composition, easier operation, and more convenient transportation
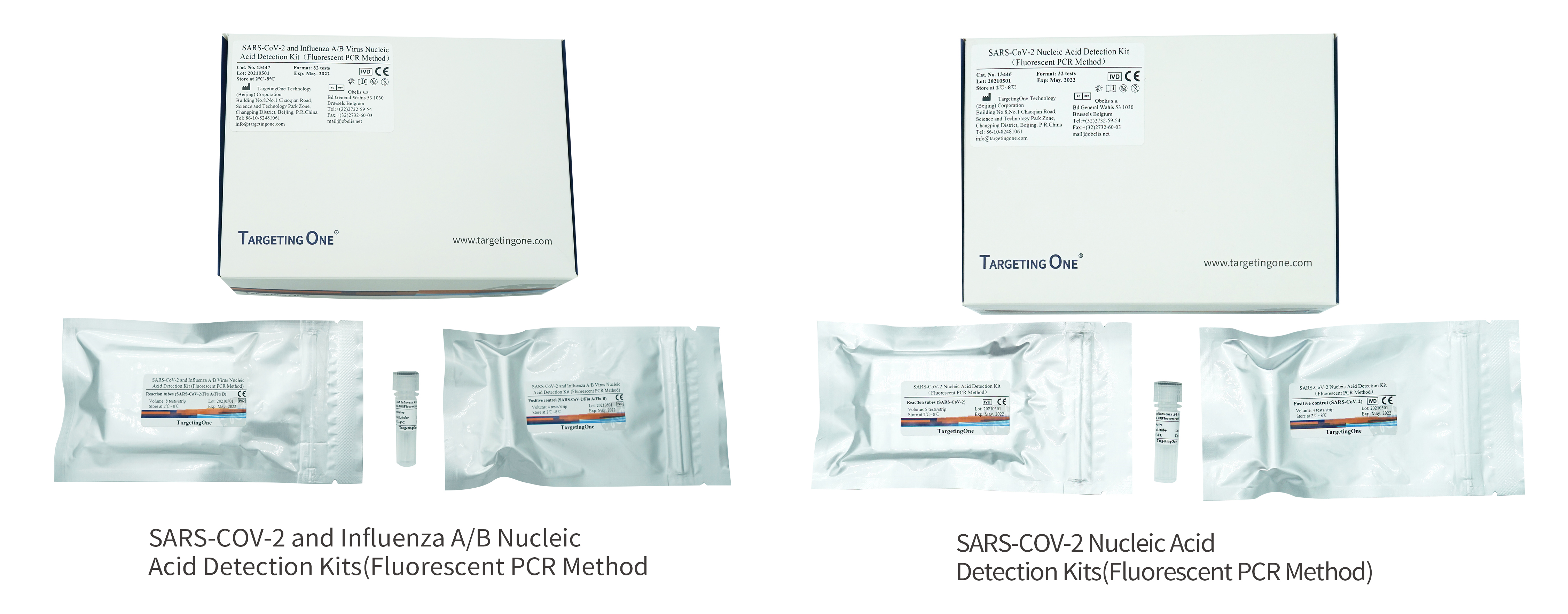
High sensitivity: the lowest detection value reaches 200 copies/mL
Applicable models: Applicable to a variety of domestic and foreign qPCR models
Anti-contamination: closed operation throughout the whole process, reducing aerosol contamination
High sensitivity: the lowest detection limit is 100 copies/mL
Accurate quantification: no need for standards and standard curves
Simple operation: one-step sample loading with low human error
Closed assay: prevent aerosol contamination and sample loss, prevent false positives
Good specificity: no cross-reactivity with other common respiratory pathogens (viruses, bacteria, fungi)

On February 3rd, 2020, novel coronavirus Detection series products (digital PCR) of TargetingOne were selected as the first batch of new anti-epidemic technology products in Zhongguancun; on March 10th, they were selected as the recommended list of medical equipment urgently needed for novel coronavirus prevention and control in China Medical Equipment Association; on March 16th, they were selected as the first batch of new technology products for epidemic prevention and control in Ministry of Science and Technology; and on April 2nd, they were granted CE market access qualification in EU.
Up to now, the whole chain of TargetingOne's novel coronavirus nucleic acid testing products: digital PCR and qPCR platforms have been qualified for CE market access in the EU, which can provide global novel coronavirus nucleic acid testing products and technical support services to help overseas epidemic prevention and control.